If you'd like to start a new discussion under Life Sciences / Pharma, go to Pharma/Life Sciences , and click "New Topic".
To follow new posts, be sure to at least click "Watching First Post" on Pharma/Life Sciences stry:pharma-life-sciences , and click "New Topic".
Recap
Here's a recap of some of the activity related to Life Sciences / Pharma at rstudio::global(20210)
Workshop - Intro to Shiny
Interested in our workshop, R in Pharma with ProCogia, Intro to Shiny? Access the workshop's resources here.
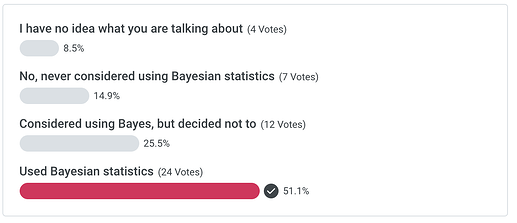
Poll - Have you ever considered using Bayesian methods in your research? And why?
Martin Modrák to Life Sciences / Pharma - January 21st, 9:06 AM
Hi, I am a bioinformatician and dabbling in biostatistics. Ask me anything about Bayesian statistics in general and Stan in particular! Have you ever considered using Bayesian methods in your research? And why?
Phil Bowsher - January 21st, 11:58 AM
Lots of interest in Stan for Nonlinear Mixed Effects Modeling, especially in PKPD. Daniel Lee (generable.com/about/) taught a Stan workshop at R in Pharma. metrumrg also has some good info on this.
Maurizio - I love Bayesian statistics and used it for my PhD research project.
I plan to start learning how to use it in R, although I'm not sure it will be useful for my job.
Phil Bowsher - For Bayesian PKPD modeling, Stan, rstan and Torsten come up.
Adam - I really like Richard McElreath's "Statistical Rethinking" book on Bayesian stats. I also found the gen package really intriguing but never used it.
Will - I love cmdstanr: light, fast, convenient. (Just built a workflow package on top of it: https://wlandau.github.io/stantargets/.) Bayes in general helps my group think about and communicate inference more straightforwardly.
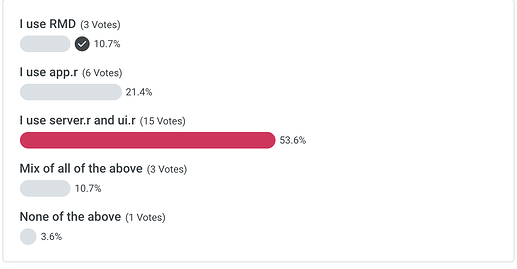
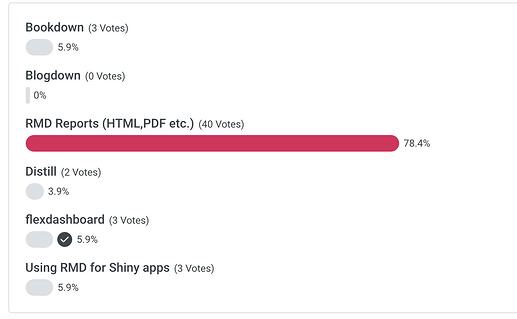
Poll - When creating a shiny app for LS work…
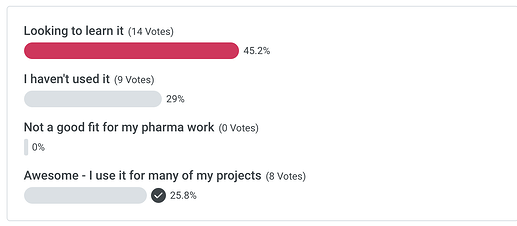
Poll - renv for your life sciences work…
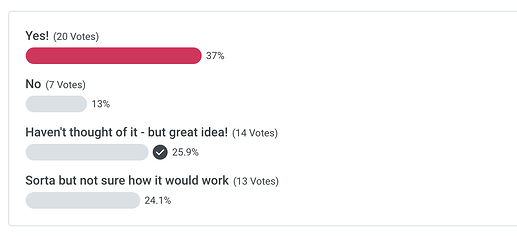
Poll - Has your team thought about using Shiny for submissions?
Aedin - What kind of submissions?
Phil Bowsher - Hi Aedin! Great question. Interest is growing in the pharma space around using shiny for regulatory submissions in electronic format, especially submitting a filing to the FDA using Clinical Data Interchange Standards Consortium(CDISC) data standards...Biogen has this app for example:
GitHub - Biogen-Inc/tidyCDISC: Demo the app here: https://bit.ly/tidyCDISC_app
Bob at Biogen gave a talk on this Tuesday at the rstudio global R/Pharma session.
Another example is here: https://williamnoble.shinyapps.io/rsum/
By William Noble at Sarah Cannon. The about page has some good info.